In the Journal of the American Chemical Society, Stylianou et al have reported on the investigation of the structural transformation of a Zn-based flexible
Measuring High Resolution Isotherms and Kinetics
Measuring high-resolution isotherms and kinetics over wide pressure ranges with XEMIS sorption analyzers
Measurements with small samples at high pressures
We reported previously on the publication of hydrogen adsorption data to 150 bar using our XEMIS high-pressure gas sorption analyzer [1]. Recently a subsequent article, published in September 2015 issue of Journal of Nano Energy, reported further hydrogen adsorption data on novel carbon materials with sample sizes as small as 15 mg [2].
The authors compare data measured with Hiden Isochema IGA analyzers to 20 bar, and with XEMIS analyzers to 150 bar, again with excellent agreement. They report H2 and CO2 uptake data along with other techniques to evaluate a ‘compactivation’ method involving mechanical compression prior to thermochemical activation, which yields carbons with high gravimetric and volumetric H2 and CO2 storage densities. Gravimetric sorption data of this quality, at high pressures, on such research-scale samples from 15 mg is uniquely possible with Hiden Isochema’s XEMIS sorption analyzer.
High-resolution measurements at low pressures
For other applications, notably those related to environmental and atmospheric sciences, it is also necessary to obtain high-resolution adsorption-desorption isotherm data at lower pressures.
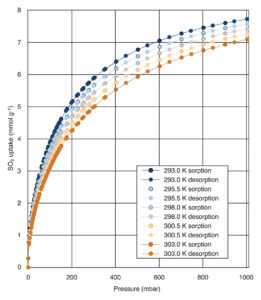
Fig.1: SO2 adsorption-desorption isotherms on activated carbon
An example of the high-resolution measurements possible with XEMIS sorption analyzers is demonstrated further by the data in Fig. 1. High-resolution SO2 adsorption-desorption isotherms on a commercial activated carbon (Filtrasorb F400) are measured at 5 equally spaced temperatures, 2.5 °C apart in the range 20 – 30 °C. The lack of hysteresis and even spacing is clear and demonstrates excellent reversibility.
Naturally, the complete kinetics are recorded simultaneously with the equilibrium data and the full raw data is readily available to users. An example of the raw data with continuous kinetic trend analysis is also shown in Fig. 2. The relevant device readings (pressure, temperature, weight) are continuously recorded at an interval specified by the user, which can be defined as frequently as 0.1 second intervals. The intrinsic long term stability of the XEMIS microbalance allows this sampling rate to be maintained for the full duration of the measurement with no breaks. The kinetics are used by the HIsorp software to determine the equilibration time required for each pressure point, using HIsorp’s intelligent real time trend analysis function. Residuals to the analysis fit are displayed in real time and the kinetics can be further analysed to yield diffusion coefficient as well as providing a direct measure of the rate of approach to equilibrium.
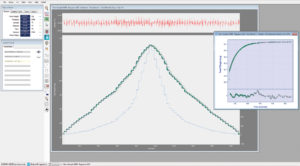
Fig. 2: Full raw data with kinetic trend analysis (inset)
To receive further information on the XEMIS, or receive a quotation, please contact us now.
[1] B. Adeniran and R. Mokaya, Journal of Materials Chemistry A 3, 5148-5161 (2015).
[2] B. Adeniran and R. Mokaya, NanoEnergy 16 (2015) 173-185.


