In the Journal of the American Chemical Society, Stylianou et al have reported on the investigation of the structural transformation of a Zn-based flexible
Understanding high pressure gas adsorption and storage in shales
In this post, we highlight a recent paper reporting the characterization of supercritical methane adsorption and storage by a series of shales and isolated kerogens, extracted from shale gas plays in both Denmark and Germany.
Shale gas has experienced a boom over the last decade or so, particularly in the US, and gas extracted from shale formations now accounts for a significant proportion of US natural gas production. Large potential shale gas reserves also exist elsewhere, and these may be exploited in the future. Understanding the adsorption of methane, the main constituent of shale gas, under geological conditions of temperature and pressure, is important in order to assess the potential capacity of shale gas plays. Determining structure-property relationships for shales, however, is challenging due to their complex and inhomogeneous nature, and their relatively low gas adsorption capacities.
Recent work by Professor Mark Thomas and co-workers has shed light on this topic, by combining different characterization techniques with high pressure manometric gas adsorption measurements [1]. High pressure methane adsorption isotherms were measured in a Hiden Isochema IMI in the temperature range 300 K to 473 K, at pressures up to 14.0 MPa (see Fig. 1). These measurements allowed the isosteric enthalpy of adsorption to be determined using the Clausius-Clapeyron equation, and this helped validate the thermodynamic consistency of the data.
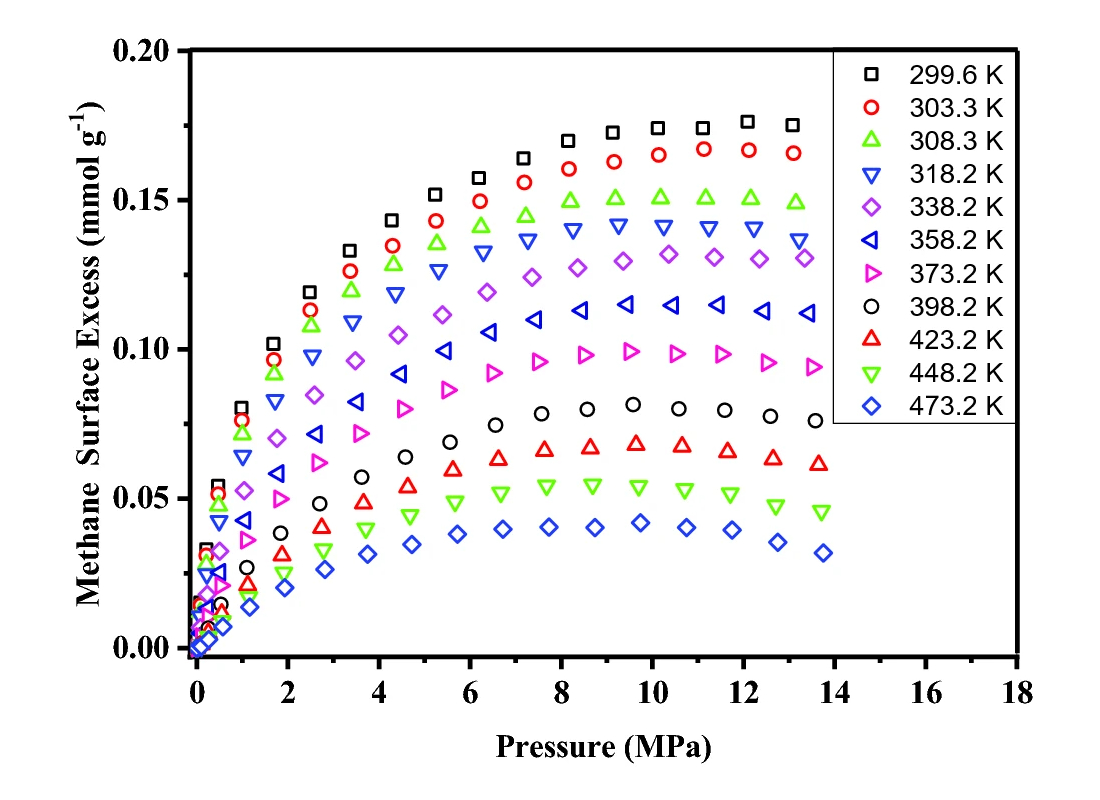
Figure 1. Supercritical methane adsorption isotherms measured on an Alum shale, obtained from the Skelbro-2 well in Bornholm, Denmark. Figure reproduced from [1], under the CC by 4.0 license.
A series of low pressure gas adsorption measurements were also made on the same sample. Figure 2 shows the adsorption of N2 at 77 K, CH4 at 112 K, and CO2 at 195 K, measured using a Hiden Isochema IGA and normalized to the saturation pressure of each species. Given the different temperatures of each measurement, the varying properties of each gas and the low uptakes exhibited by shales, the agreement is excellent. It also demonstrates that measuring CO2 adsorption at the more experimentally convenient temperature of 195 K can be used to assess the pore volume of the material accessible to CH4. This provides an estimate of the maximum volume available for methane adsorption and hence the maximum storage capacity of the material, excluding compressed gas in larger pores.
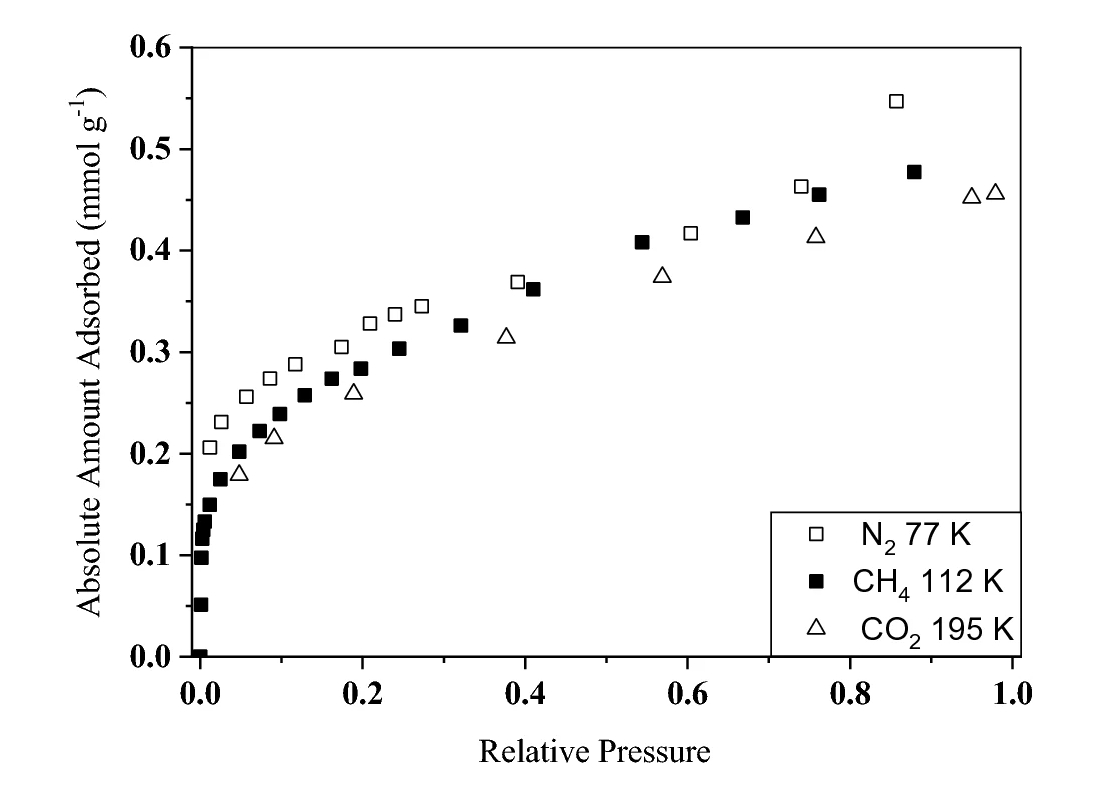
Figure 2. Subcritical gas adsorption isotherms measured using N2 at 77 K, CH4 at 112 K, and CO2 at 195 K on an Alum shale, obtained from the Skelbro-2 well in Bornholm, Denmark. Figure reproduced from [1], under the CC by 4.0 license.
A range of other characterization techniques were also applied in the study, including pycnometry, mercury porosimetry, and scanning electron microscopy (SEM). A range of techniques are required due to the heterogeneity of shales and the complementary information provided by each approach. Mercury porosimetry, for example, probes larger pore sizes than low pressure gas adsorption. Figure 3 summarizes the different ranges of porosity probed by various techniques.
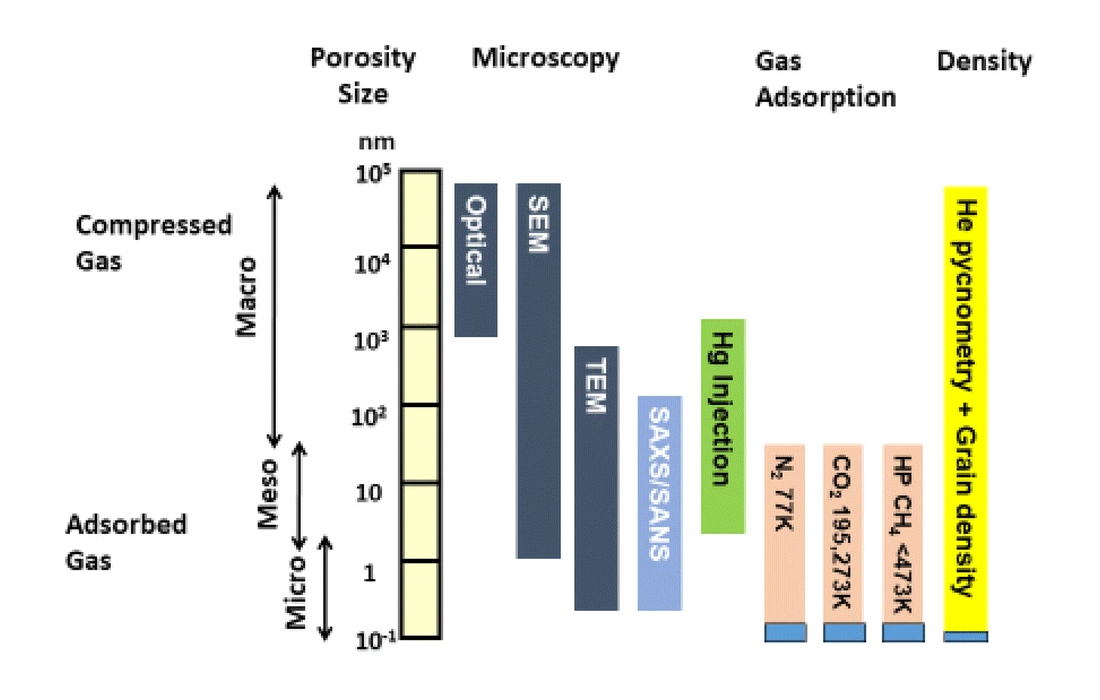
Figure 3. The various pore sizes probed by different experimental techniques used for shale characterization. Figure reproduced from [1], under the CC by 4.0 license.
Figure 4 illustrates how the subcritical gas adsorption measurements used in the study can be combined to obtain information related to high pressure supercritical methane adsorption by shales, to allow thorough characterization of their natural gas storage properties under practical conditions.
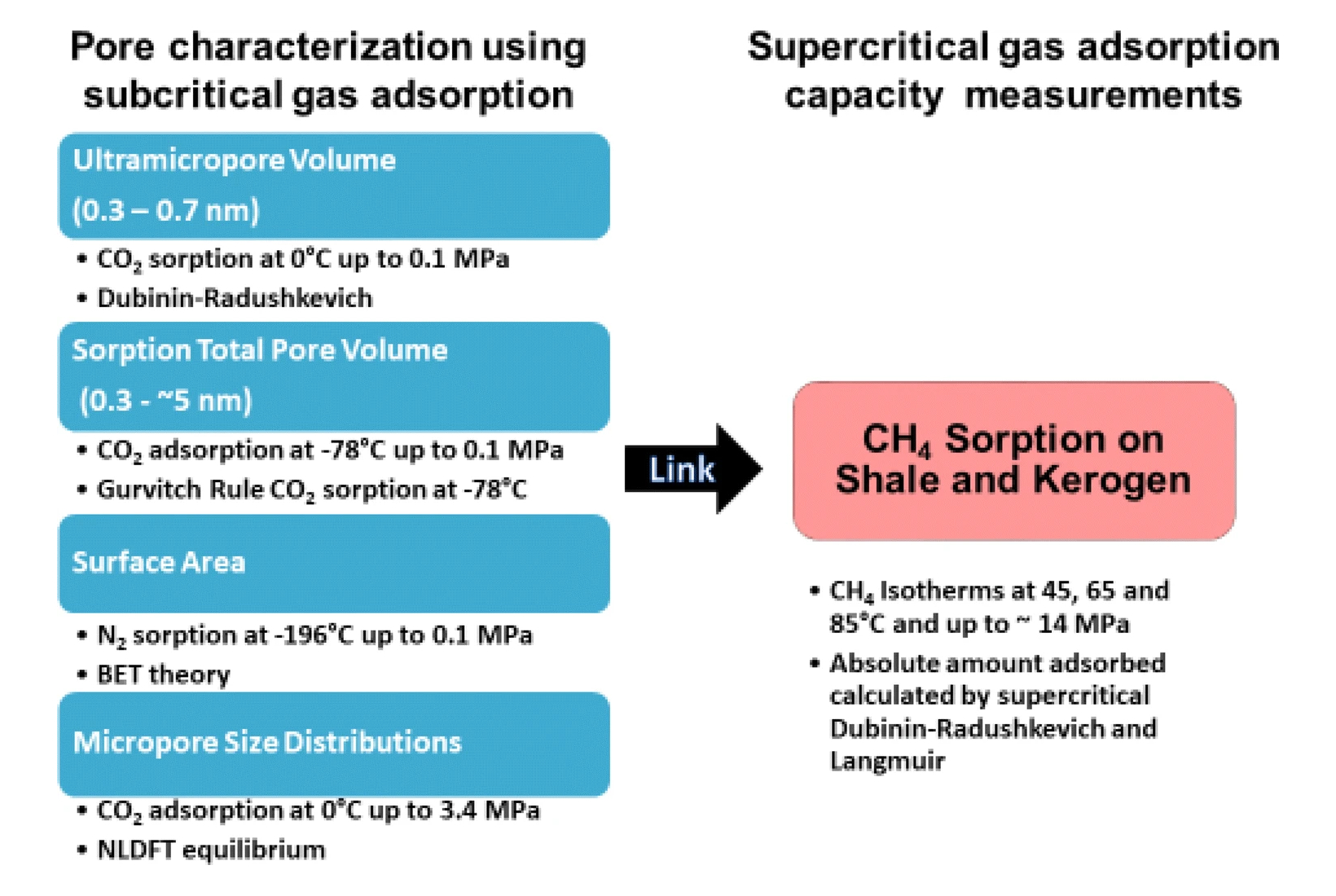
Figure 4. A summary of the various properties obtained from subcritical gas adsorption measurements, which can be combined with high pressure supercritical methane adsorption measurements, to characterize shales. Figure reproduced from [1], under the CC by 4.0 license.
The authors then applied a similar approach to a series of Posidonia shales and isolated kerogens, obtained from the Wickensen (WIC), Harderode (HAR), and Haddessen (HAD) boreholes in Germany, and were able to establish a linear relationship between CO2 pore volume and high pressure excess CH4 adsorption capacity, as shown in Fig. 5.
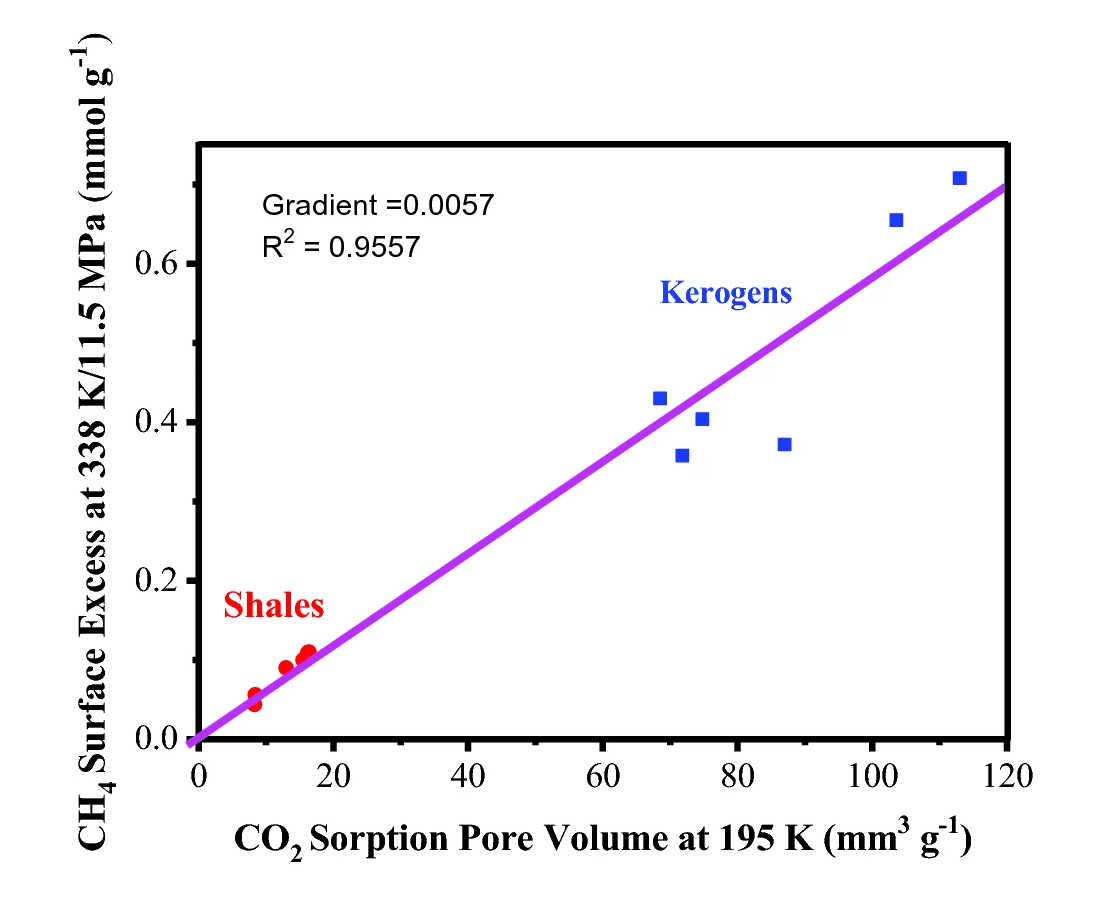
Figure 5. The relationship between high pressure supercritical excess CH4 adsorption capacity and the CO2 pore volume measured at 195 K, for a series of Posidonia shales and isolated kerogens. Figure reproduced from [1], under the CC by 4.0 license.
This study therefore offers considerable insight into the relationship between the porous properties of shales and how characterization using subcritical gas adsorption can complement high pressure gas adsorption experiments to assess storage capacities. Further details can be found in the full paper [1], which is freely available to download from the SpringerLink website.
If you would like further information about Hiden Isochema instruments, please do not hesitate to contact us.
References
[1] Rexer, T. F., Mathia, E. J., Aplin, A. C., Thomas, K. M. (2020) Supercritical methane adsorption and storage in pores in shales and isolated kerogens. SN Applied Sciences 2, 780


