Measuring ethyl acetate vapor sorption selectivity in porous materials
Hiden Isochema scientists recently co-authored a paper, published in Chemistry – A European Journal, reporting the selective adsorption of ethyl acetate by a porous macrocycle molecule.
The study, led by Professor Andy Cooper and Dr. Ming Liu at the University of Liverpool, also included contributions from the University of Southampton and East China University of Science and Technology.
Ethyl acetate (EA) is widely used as a solvent in the chemical industry and is primarily synthesized from ethanol (EtOH). Separation of the product EA from EtOH is challenging and energetically demanding, as the boiling points of the two species are very close. Adsorption-based separation of EA from EtOH using microporous materials is therefore an interesting and industrially relevant research area.
The study reports the synthesis and structural analysis of an ethyl acetate solvent templated trianglimine macrocycle (TAMC), and the macrocycle’s EA and EtOH vapor phase adsorption behavior. Vapor phase adsorption isotherms, kinetics and cyclic adsorption-desorption measurements were measured using an IGA-002 gravimetric sorption analyzer and breakthrough measurements recorded using an ABR automated breakthrough analyzer.
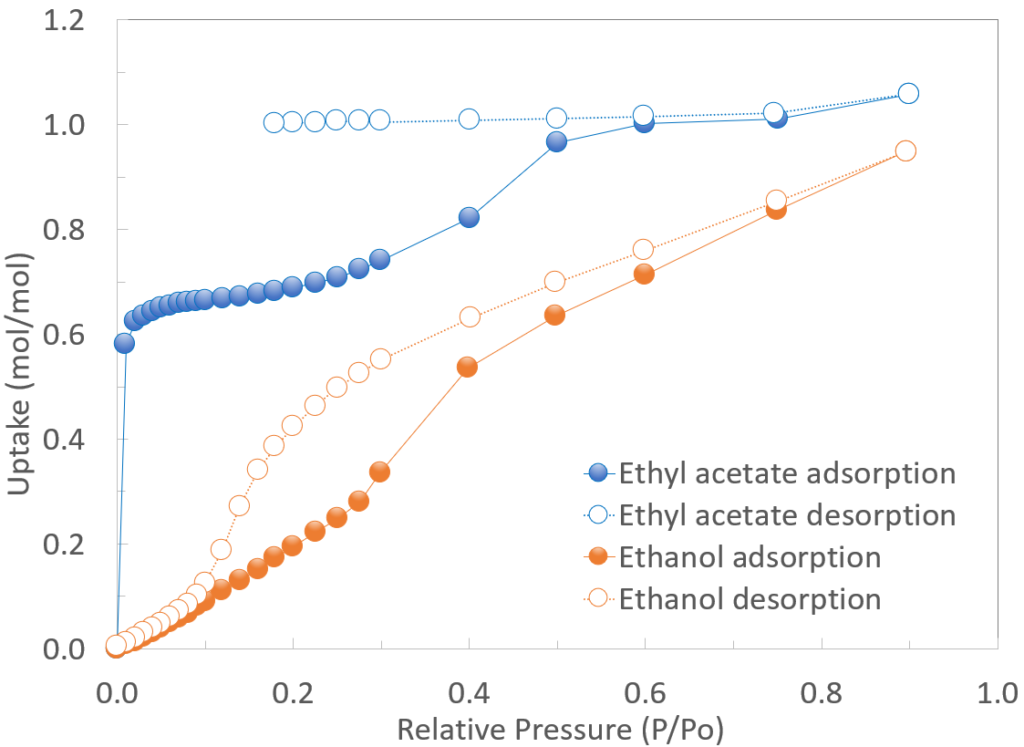
Ethyl acetate vapor sorption isotherm and kinetic measurements for TAMC at 298 K showed high vapor sorption uptake at very low pressures, and a complex isotherm shape, with equimolar uptake above P/Po = 0.5, in good agreement with structural results. EA desorption exhibited remarkable hysteresis and very slow kinetics, due to the strong adsorbent-adsorbate interactions. In contrast, the EtOH isotherm under equivalent conditions showed more gradual sorption uptake as a function of vapor pressure, faster desorption kinetics and a much lower extent of hysteresis. The IGA-002 was also used to study cyclic EA sorption-desorption behaviour for TAMC. Five cycles were measured with the adsorption conditions being 13.33 kPa at 25 °C, and desorption conditions being high vacuum (1×10-4 Pa) at 70 °C. Each adsorption cycle resulted in equimolar uptake at equilibrium, and each desorption cycle was sufficient to rapidly and completely desorb EA.
An ABR with integrated vapor generator was used to study breakthrough of EA and EtOH through a packed TAMC bed at 298 K. The vapor mixture was generated from a 1:1 v/v mixture of EA and EtOH in the ABR’s vapor generator and detected using an integrated Hiden mass spectrometer. EtOH vapor broke through the bed significantly earlier than EA, providing further direct evidence for the macrocycle’s selectivity for EA over EtOH.
Speaking about the research, Dr. Ming Liu said, “The data from Hiden Isochema’s gravimetric sorption and breakthrough analyzers provided us with conclusive experimental evidence regarding the high selectivity of our material.”
To learn more about Hiden Isochema’s gravimetric and breakthrough sorption analyzers, please get in touch.
Reference:
Inherent Ethyl Acetate Selectivity in a Trianglimine Molecular Solid
D. He, C. Zhao, L, Chen, M. A. little, S. Y. Chong, R. Clowes, K. McKie, M. G. Roper G. M. Day, M. Liu and A. I. Cooper, Chem. Eur. J., 2021. DOI: 10.1002/chem.202101510.


