In the Journal of the American Chemical Society, Stylianou et al have reported on the investigation of the structural transformation of a Zn-based flexible
Measuring the adsorption of corrosives with the XEMIS
In a previous blog item we noted the broad interest in the determination of the adsorption of different gases and vapors by materials. Many areas of scientific research and engineering can make use of this type of information. However, the practical adsorption properties of a material can also be exploited to solve problems in gas separation and purification, pollution control, and other related technologies; understanding adsorption behavior is clearly crucial to the development of both new materials and processes in these areas.
Some gases and vapors are comparatively easy to work with. They are relatively inert and unreactive. Most of the atmospheric gases, for example, nitrogen, argon, carbon dioxide, neon, helium and methane, together with water, are non-toxic.
Others, however, are more harmful, and a significant number of different species are considered to be toxic air contaminants. It is important to be able to control, reduce or eliminate the release of many of these into the environment and adsorption is one of the methods that can be used for this purpose.
Materials compatibility issues
For some of these species, their reactive nature can lead to materials compatibility issues in analytical equipment. Their handling thus involves careful consideration not only of the safety aspects of these potentially dangerous species but also their interaction with any components with which they may come into contact. Some very useful chemical compatibility tables are available online and even a brief browse through these resources reveals what a complex issue this can be.
With regard to sorption analysis, the key point is to ensure that sensitive components in the sorption apparatus are not exposed to species that could potentially cause them damage. For gravimetric instrumentation, which is the most accurate measurement option (particularly under low pressure conditions), it is the microbalance that can be most sensitive. It may therefore be necessary to remove these components from the balance chamber.
In this respect, the new Hiden Isochema XEMIS sorption microbalance represents a significant advance. The idea, in this case, is to retain the advantages of beam balances, including their high inherent stability, but to remove from the balance chamber the sensing and control mechanisms that are required for the operation of the balance. We call this exosensing technology.
Sulfur dioxide
One of the species that is more challenging to work with is sulfur dioxide, even though its study is practically very important. There is, for example, a significant amount of interest in its removal from flue gases (e.g. Flue Gas Desulfurization or FGD). This is because it is a pollutant. Fortunately, increasingly stringent environmental regulations have continued to drive down emission levels but this has also led to continued interest in the development of more effective and efficient methods of SO2 removal.
A useful collection of information regarding the properties of SO2 and its effect on human health and the environment can be found on the US Environmental Protection Agency website. An interesting report on the emissions of the main air pollutants, meanwhile, including SO2, here in the UK, produced by the Department for Environment, Food & Rural Affairs (DEFRA), can be found on the www.gov.uk website.
Due to the intense interest in the study of the interaction of SO2 with materials, for the control of its release into the environment, in particular, it is important to be able to accurately measure its uptake by new materials. At the same time, SO2 adsorption provides an excellent opportunity for us to demonstrate the capabilities of our new XEMIS microbalance for use with corrosive species. We therefore selected SO2 adsorption as one of the first sets of demonstration data for the instrument.
The SO2 adsorption and desorption isotherms measured on a Metal-Organic Framework (MOF) material, NOTT-300, up to atmospheric pressure are shown below. Measurements were made at five temperatures from 298 K to 348 K, as indicated in the figure.
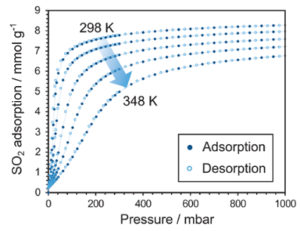
Sulfur dioxide adsorption and desorption by a metal-organic framework, courtesy of the University of Nottingham, UK
In future blog items we hope to expand further on the applications and capabilities of the new XEMIS sorption microbalance. In the meantime, if you would like to learn more about its capabilities or would like to discuss your application requirements in more detail, please do not hesitate to contact us.


